How the ELIAS Cancer Immunotherapy Works
The ELIAS cancer immunotherapy (ECI®) platform offers a completely new approach to cancer treatment. It is built upon 50+ years of intensive scientific and medical research into the interactions between cancer and the immune response. ECI utilizes a combination of cancer vaccination pretreatment and activated “killer” T cell immunotherapy.
Two-step Treatment. Explained.
Cancer treatment with ECI® involves the following sequential and dependent steps:
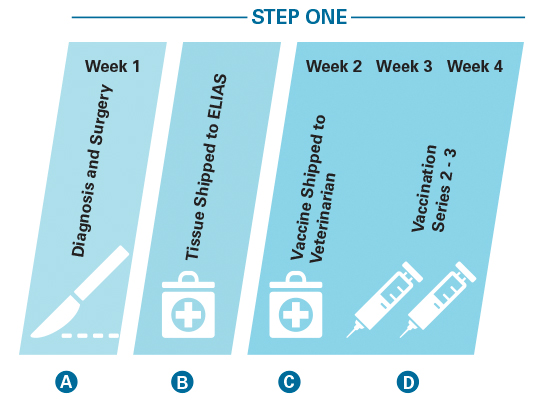
Step One: Personalized vaccine stimulates a cancer-specific immune response.
A. Cancer tissue is surgically removed by the veterinarian.
B. This tissue is shipped to ELIAS Animal Health’s manufacturing facility.
C. A personalized vaccine is produced and sent back to the veterinarian.
D. The vaccines are administered on a weekly basis to stimulate the immune system T cells to recognize the dog’s cancer.
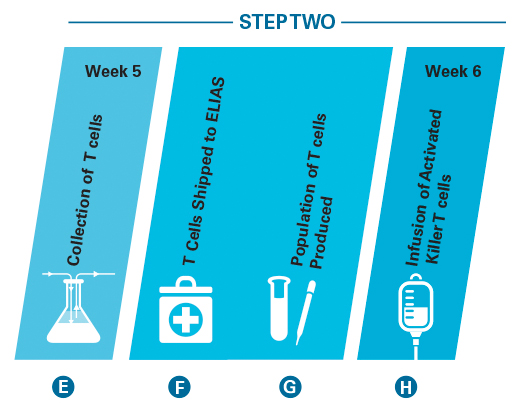
Step Two: T Cell Harvest and Reinfusion.
E. Two weeks following step one, the veterinary oncologist uses apheresis to harvest the population of cancer-specific T cells generated by the vaccination.
F. These T cells are shipped to ELIAS for processing.
G. A proprietary, patented process is used to produce an activated, blood-derived population of T cells functionally empowered and numerically expanded.
H. This tumor-specific population of killer T cells is returned to the veterinarian for intravenous administration.
Therapeutic Potential. Amplified.
ECI® is a promising oncology treatment modality for canine cancer because:
- It delivers the potential for tumor-specific cytotoxicity.
- Clinical outcomes of ELIAS’ osteosarcoma trial, as reported at ACVIM in June 2019, showed adverse events were mostly mild to moderate and were typically transient in nature.
- This personalized approach could be useful for treating numerous types of cancer and may limit or avoid the use of chemotherapy and radiation.